| Sugar - Coating - Rockefeller Univ |
By Rockefeller University |
"Sugar-coating" on proteins may safeguard body against further insult. M.D.-Ph.D. student show mannose receptors performs clearance role.
Much
like a cadre of emergency workers at the scene of an accident, the
body's immune system cells gather at the site of an injury, whether it
is a simple cut or an infection. This micro-scopic crowd largely
consists of inflammatory cells and proteins, and together they marshal
the immune system's arsenal to bring the offending stimulus under
control.
And as so often occurs at the scene of an accident, the
crowd lingers. Initially helpful, its presence can create new problems.
At the scene of a car accident, the problem may be spectators blocking
the travel of the ambulance. In the body, the crowd of inflammatory
proteins and other cells can continue to attract more immune system
agents than needed. To prevent such an uncontrolled response, a clean-up
mechanism is needed to signal the end of the rescue operation.
Now,
for the first time, Rockefeller researchers have found that a receptor
protein called the mannose receptor on liver endothelial cells performs
such a function, which may be important in preventing further damage to
healthy tissues.
Sena Lee, a biomedical fellow in the
Tri-institutional M.D.-Ph.D. Program, published part of her thesis
research on the clearance activity of the mannose receptor in the March 8
issue of Science. Michel Nussenzweig, Howard Hughes Medical Institute
investigator and head of the Laboratory of Molecular Immunology, is
Lee's advisor and a co-author of the publication.
Like emergency
workers who wear similar garb so that they may be easily identified,
many inflammatory proteins congregating at injury sites in the body
carry similar tags made of sugars. "In this setting, the sugar tag,
called high-mannose glycan, is a marker for the proteins' identity,"
says Lee. The mannose receptor recognizes high-mannose glycan as a
disposal tag. When the body no longer needs the proteins in the area,
mannose receptor initiates their removal. The receptor is then down
regulated, or turned off, until it is needed to perform the next job.
Lee
and her colleagues' research is part of the larger field of
"glyco-biology" - the chemistry and biology of carbohydrates, or sugars -
which was described in a special issue of Science in 2001.
An
editorial in the magazine called glycobiology" à Cinderella field: an
area that involves much work but, alas, does not get to show off at the
ball with her cousins, the genomes, and proteins." However, the Science
contributors suggested, more and more guests at the ball will learn
to recognize Cinderella. Glycobiology, including the research of Lee
and her colleagues, is poised to receive well-deserved attention.
"Most
biologists are interested in genes and proteins and their regulation,
not in sugars. But many proteins, especially extracellular and cell
membrane proteins, have sugars on them," says Lee. Some researchers have
made medical progress by homing in on glycans. A recent development in
HIV experimental therapy, for example, exploits the virus's dependence
on its glycoprotein shell binding to a human cell surface receptor,
CCR5.
"Sugar-coated" proteins, called glycoproteins, are involved
in myriad activities, from regulating the immune system cells' movements
and stability, to cloaking cancer cells as they navigate immunologic
defenses.
"We know that many protein-protein interactions are
based on glycans, but even more interesting, glycans are able to
directly change the con-formation or activation level of their proteins.
These modifications may be the key to understanding the functional
regulation of the body's proteins," says Lee.
Using mice that were
genetically deficient in the mannose receptor, Lee and her colleagues
identified all the sugars that could bind to the receptor. They then
induced inflammation in the normal, or wild type, and the knockout, or
mutant, mice.
The experiments were designed to reveal whether the
mannose receptor removes a class of circulating glyco-proteins from
blood, and whether there is another bodily clearance mechanism if the
receptor is lacking or disabled. The mutant mice could not clear the
glycoproteins released during inflammation.
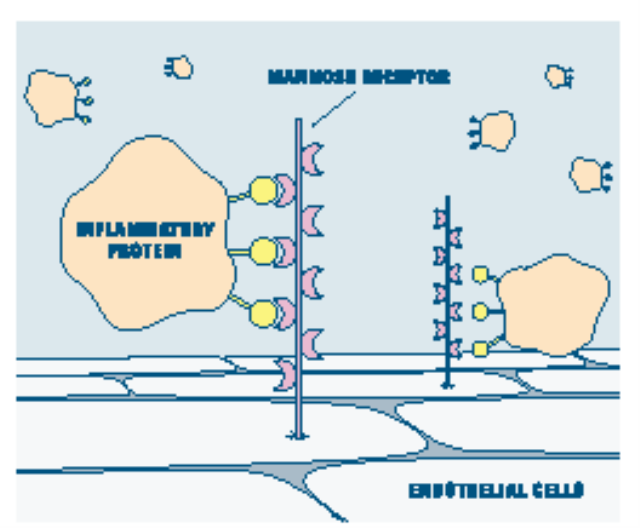

Inflammatory
proteins are helpful in repairing an injury inside the body, but are no
longer needed when the crisis has passed. Their presence after an
injury can harm surrounding tissues. To dispose of them, mannose
receptors (violet) are turned on and bind with the complex sugars
(yellow) tagging the proteins. The anchoring endothelial cell then
engulfs the bound proteins.
These findings indicate that the body
efficiently regulates inflammatory proteins by coating them with
glycans and by using receptors like the mannose receptor that recognize
these glycans to retrieve the proteins when they are no longer useful.
"Sena's
work is exciting because no one has ever proven that blood protein
levels can be regulated by a glycan receptor this way," says
Nussenzweig.
More research is required to fully understand the
role of glycans and their receptors in the regulation of inflammation,
but one potential avenue for biomedical applications is in
pharmacokinetics. Manipulating the levels of certain proteins in the
body could be achieved by altering the glycans they carry. In this way,
scientists could design ways to limit or extend the circulatory lifespan
of bioactive proteins.
Lee will bring laboratory research to bear
on the medical practice she establishes in the future, but first she
must complete her medical training. Glycobiology, the so-called
"Cinderella field," will have to wait for Lee to return to the ball. -
Lynn Love
Courtesy: The Rockefeller Univ. Newsletter
"News & Notes" Friday, April 12, 2002
The product
statements have not been evaluated by FDA and the products are not intended to
diagnose, treat, cure or prevent any disease or medical condition. Contents of
this website are for informational purposes should not be used for diagnosing
or treating a health problem or disease.
|